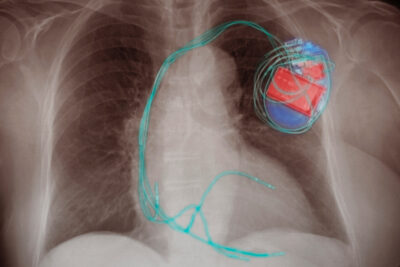
Hundreds of thousands of people rely on lifesaving medical devices, from pacemakers and defibrillators to implanted prosthetics. The U.S. regulatory system is supposed to protect all of them from unsafe devices and unscrupulous actors.
But our latest investigation into the $400 billion medical device industry showed that, thanks to ineffective oversight, vulnerable people may be getting hurt. We uncovered that the FDA took no decisive action as a heart pump was implanted inside thousands of people, even though the agency knew it didn’t meet federal standards.
Now, we need your help continuing to hold medical device companies and regulatory systems accountable. We want to hear from people who know this system best: patients, doctors, people who work for device companies and federal employees. We’re particularly interested in device marketing and communication around recalls. If you have insights that could help guide our reporting, or if you know about other problems we should investigate, please fill out our brief questionnaire.